Tutorial: Adding a Latent Stage to Make an SEIR Model
Day 4 | Network Modeling for Epidemics
EpiModel includes an integrated SIR model, but here we show how to model an SEIR disease like COVID-19. The E compartment in this disease is an exposed state in which a person has been infected but is not infectious to others. Some infectious diseases have this latent non-infectious stage, and in general it provides a general framework for transmission risk that is dependent on one’s stage of disease progression.
Setup
First start by loading EpiModel and clearing your global environment.
library(EpiModel)
rm(list = ls())EpiModel Model Extensions
Conceptualization
The first step to any EpiModel extension model is to conceptually identify what new functionality, above and beyond the built-in models, is desired and where that functionality should be added. There are often many “right” answers to these questions, and this aspect is only learned over time. But in general, it is helpful to map out a model extension on a state/flow diagram to pinpoint where the additions should be concentrated.
SIR Module Set
In this particular model, we will be adding a new disease state that
occurs in between the susceptible disease state and an infectious
disease state in an SIR model. The standard, built-in SIR model in
EpiModel uses set modules (elements of the model), each with its own set
of associated functions (the realization of those elements in code). For
any built-in model, you can see what set of modules and functions have
been used by running the simulation with netsim and then
printing the output.
nw <- network_initialize(n = 100)
formation <- ~edges
target.stats <- 50
coef.diss <- dissolution_coefs(dissolution = ~offset(edges), duration = 20)
est1 <- netest(nw, formation, target.stats, coef.diss, verbose = FALSE)
param <- param.net(inf.prob = 0.3, rec.rate = 0.1)
init <- init.net(i.num = 10, r.num = 0)
control <- control.net(type = "SIR", nsteps = 25, nsims = 1, verbose = FALSE)
mod1 <- netsim(est1, param, init, control)
mod1EpiModel Simulation
=======================
Model class: netsim
Simulation Summary
-----------------------
Model type: SIR
No. simulations: 1
No. time steps: 25
No. NW groups: 1
Fixed Parameters
---------------------------
inf.prob = 0.3
rec.rate = 0.1
act.rate = 1
groups = 1
Model Output
-----------------------
Variables: s.num i.num r.num num si.flow ir.flow
Networks: sim1
Transmissions: sim1
Formation Diagnostics
-----------------------
Target Sim Mean Pct Diff Sim SD
edges 50 41.32 -17.36 3.637
Dissolution Diagnostics
-----------------------
Target Sim Mean Pct Diff Sim SD
edges 0.05 NaN NaN NAModule Classes
This includes a series of modules, which we classify into
Standard and Flexible modules as shown
on the table below. Any modules may be modified, but standard modules
are those that are typically not modified because they generalize the
core internal processes for simulation object generation, network
resimulation, and epidemic bookkeeping. Flexible modules, in contrast,
are those that are likely necessary to modify for an EpiModel extension.
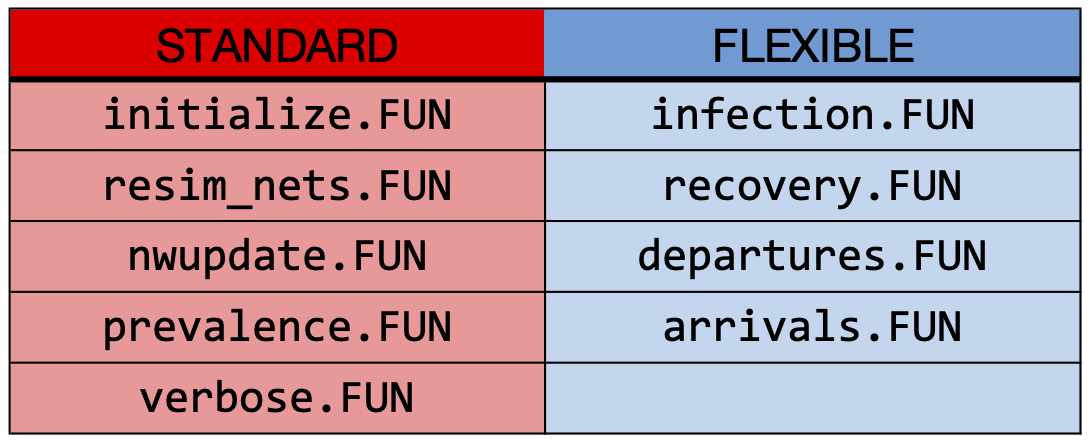
In the example above, we used all of these modules except the arrivals and departures modules because we had a closed population. In addition to these set of built-in modules, any user can add more modules to this set, depending on what is needed. Again, this involves some conceptualization of how to organize the model processes, including whether those processes are similar to the built-in modules or something new.
Modules vs. Functions
All modules have associated functions, and these are passed into the
epidemic model run in netsim through
control.net. Printing out the arguments for this function,
you will see that each of the standard modules have default associated
functions as inputs, and flexible modules have a default of
NULL.
args(control.net)function (type, nsteps, start = 1, nsims = 1, ncores = 1, resimulate.network = FALSE,
tergmLite = FALSE, cumulative.edgelist = FALSE, truncate.el.cuml = 0,
attr.rules, epi.by, initialize.FUN = initialize.net, resim_nets.FUN = resim_nets,
infection.FUN = NULL, recovery.FUN = NULL, departures.FUN = NULL,
arrivals.FUN = NULL, nwupdate.FUN = nwupdate.net, prevalence.FUN = prevalence.net,
verbose.FUN = verbose.net, module.order = NULL, save.nwstats = TRUE,
nwstats.formula = "formation", save.transmat = TRUE, save.network,
save.other, verbose = TRUE, verbose.int = 1, skip.check = FALSE,
raw.output = FALSE, tergmLite.track.duration = FALSE, set.control.ergm = control.simulate.formula(MCMC.burnin = 2e+05),
set.control.stergm = NULL, set.control.tergm = control.simulate.formula.tergm(),
...)
NULLFor built-in models, EpiModel selects which modules are needed based
on the model parameters and initial conditions. For each time step, the
modules run in the order in which they are specified in the output here;
this also matches the order in which they are listed in
control.net.
Built-in Functions as Templates
Any of the built-in functions associated with flexible modules are
intended to be templates for user inspection and extension for
research-level models. So, for example, mod1 above shows
that infection.net was used as the infection module. That
function has a help page briefly describing what it does. And you can
also inspect the function contents with:
View(infection.net)We’ll use an edited down version of that function, with some
additional explanation, below. In addition, the disease progression
state transition within an SIR model (and an SIS model too) is handled
by the recovery module, with an associated recovery.net
function.
View(recovery.net)We will use an edited down version of that function as a template for a new, more general disease progression module.
The EpiModel API
All EpiModel module functions have a set of shared design requirements. This set of requirements defines the EpiModel Application Programming Interface (API) for extension. The best way to learn this is through a concrete example like this, but here are the general API rules:
- Each module has an associated R function, the general design of which is:
fx <- function(dat, at) {
## function processes that update dat
return(dat)
}The function takes an object called dat, which is the
master data object passed around by netsim, performs some
processes (e.g., infection, recovery, aging, interventions), updates the
dat object, and then returns that object. The other input
argument to each function must be at, which is a time step
counter.
Data are stored on the
datobject in a particular way: in sublists that are organized by category. The main categories of data to interact with include model inputs (parameters, initial conditions, and controls) from those three associated input functions; nodal attributes (e.g., an individual disease status for each person); and summary statistics (e.g., the disease prevalence at at time step). There are accessor functions for reading (these are theget_functions) and writing (these are theset_functions) to thedatobject in the appropriate place.The typical function design involves three steps: a) reading the relevant inputs from the
datobject; b) performing some micro-level process on the nodes that is usually a function of fixed parameters and time-varying nodal attributes; c) writing the updated objects back on to thedatobject.
Let’s see how this API works by extending our infection and recovery functions to transition an SIR model into an SEIR model.
Infection Module
The built-in infection module for an SIR model performs the following
functions listed in the function below. The core process is determining
which edges are eligible for a disease transmission to occur, and then
randomly simulating that transmission process. Why is it necessary to
update the infection function for an SEIR model? Because an SIR involves
a transition between S and I disease statuses when a new infection
occurs, but an SEIR model involves a transition between S and E disease
statuses. It’s a small, but important change. Here’s the full modified
function, with embedded comments. Note that we can use the
browser function to run this function in debug mode by
uncommenting the third line (we will demonstrate this).
infect <- function(dat, at) {
## Uncomment this to run environment interactively
# browser()
## Attributes ##
active <- get_attr(dat, "active")
status <- get_attr(dat, "status")
infTime <- get_attr(dat, "infTime")
## Parameters ##
inf.prob <- get_param(dat, "inf.prob")
act.rate <- get_param(dat, "act.rate")
## Find infected nodes ##
idsInf <- which(active == 1 & status == "i")
nActive <- sum(active == 1)
nElig <- length(idsInf)
## Initialize default incidence at 0 ##
nInf <- 0
## If any infected nodes, proceed with transmission ##
if (nElig > 0 && nElig < nActive) {
## Look up discordant edgelist ##
del <- discord_edgelist(dat, at)
## If any discordant pairs, proceed ##
if (!(is.null(del))) {
# Set parameters on discordant edgelist data frame
del$transProb <- inf.prob
del$actRate <- act.rate
del$finalProb <- 1 - (1 - del$transProb)^del$actRate
# Stochastic transmission process
transmit <- rbinom(nrow(del), 1, del$finalProb)
# Keep rows where transmission occurred
del <- del[which(transmit == 1), ]
# Look up new ids if any transmissions occurred
idsNewInf <- unique(del$sus)
nInf <- length(idsNewInf)
# Set new attributes and transmission matrix
if (nInf > 0) {
status[idsNewInf] <- "e"
infTime[idsNewInf] <- at
dat <- set_attr(dat, "status", status)
dat <- set_attr(dat, "infTime", infTime)
dat <- set_transmat(dat, del, at)
}
}
}
## Save summary statistic for S->E flow
dat <- set_epi(dat, "se.flow", at, nInf)
return(dat)
}Each step is relatively self-explanatory with the comments, but we will run this interactively at the end of this tutorial to step through the updated data structures. Infection is one of the more complex processes because it involves a dyadic process (a contact between an S and I node). That involves a construction of a discordant edgelist; that is, a list of edges in which there is a disease discordant dyad.
The main point here is: we have made a change to the infection module
function, and it consists of updating the disease status of a newly
infected persons to "e" instead of "i".
Additionally, we are tracking a new summary statistic,
se.flow that tracks the size of the flow from S to E based
on the number of new infected, nInf at the time step.
Progression Module
Next up is the disease progression module. Here, we have generalized
the built-in recovery module function to handle two disease progression
transitions after infection: E to I (latent to infectious stages) and I
to R (infectious to recovered stages). Like many individual-level
transitions, this involves flipping a weighted coin with
rbinom: this performs a series of random Bernoulli draws
based on the specified parameters. Here is the full model function.
progress <- function(dat, at) {
## Uncomment this to function environment interactively
# browser()
## Attributes ##
active <- get_attr(dat, "active")
status <- get_attr(dat, "status")
## Parameters ##
ei.rate <- get_param(dat, "ei.rate")
ir.rate <- get_param(dat, "ir.rate")
## E to I progression process ##
nInf <- 0
idsEligInf <- which(active == 1 & status == "e")
nEligInf <- length(idsEligInf)
if (nEligInf > 0) {
vecInf <- which(rbinom(nEligInf, 1, ei.rate) == 1)
if (length(vecInf) > 0) {
idsInf <- idsEligInf[vecInf]
nInf <- length(idsInf)
status[idsInf] <- "i"
}
}
## I to R progression process ##
nRec <- 0
idsEligRec <- which(active == 1 & status == "i")
nEligRec <- length(idsEligRec)
if (nEligRec > 0) {
vecRec <- which(rbinom(nEligRec, 1, ir.rate) == 1)
if (length(vecRec) > 0) {
idsRec <- idsEligRec[vecRec]
nRec <- length(idsRec)
status[idsRec] <- "r"
}
}
## Write out updated status attribute ##
dat <- set_attr(dat, "status", status)
## Save summary statistics ##
dat <- set_epi(dat, "ei.flow", at, nInf)
dat <- set_epi(dat, "ir.flow", at, nRec)
dat <- set_epi(dat, "e.num", at,
sum(active == 1 & status == "e"))
dat <- set_epi(dat, "r.num", at,
sum(active == 1 & status == "r"))
return(dat)
}This set of two progression processes involves querying who is
eligible to transition, randomly transitioning some of those eligible,
updating the status attribute for those who have
progressed, and then recording some new summary statistics.
Network Model
With the epidemic modules defined, we will now step back to
parameterize, estimate, and diagnose the TERGM. Here we will use a
relatively basic model with an edges and
degree(0). Here we are not using any nodal attributes in
either the TERGM or the epidemic modules, but these could be added (we
will get more practice with that tomorrow). Note that we are using a
relatively high mean degree (2 per capita) compared to some of our prior
models, with lower than expected isolates.
# Initialize the network
nw <- network_initialize(500)
# Define the formation model: edges + degree terms
formation = ~edges + degree(0)
# Input the appropriate target statistics for each term
target.stats <- c(500, 20)
# Parameterize the dissolution model
coef.diss <- dissolution_coefs(dissolution = ~offset(edges), duration = 25)
coef.dissDissolution Coefficients
=======================
Dissolution Model: ~offset(edges)
Target Statistics: 25
Crude Coefficient: 3.178054
Mortality/Exit Rate: 0
Adjusted Coefficient: 3.178054Next we fit the network model.
# Fit the model
est <- netest(nw, formation, target.stats, coef.diss)And then diagnose it. We are including a wide range of degree terms
to monitor so we can see the full degree distribution . Although the
degree(0) term does not look great visually, it is only off
by 2-3 edges in absolute terms.
dx <- netdx(est, nsims = 10, ncores = 5, nsteps = 500,
nwstats.formula = ~edges + degree(0:7),
keep.tedgelist = TRUE)
Network Diagnostics
-----------------------
- Simulating 10 networks
- Calculating formation statistics
- Calculating duration statistics
- Calculating dissolution statistics
print(dx)EpiModel Network Diagnostics
=======================
Diagnostic Method: Dynamic
Simulations: 10
Time Steps per Sim: 500
Formation Diagnostics
-----------------------
Target Sim Mean Pct Diff Sim SD
edges 500 491.825 -1.635 15.651
degree0 20 26.292 31.462 5.264
degree1 NA 179.999 NA 11.285
degree2 NA 153.414 NA 10.320
degree3 NA 86.806 NA 8.769
degree4 NA 36.757 NA 6.014
degree5 NA 12.275 NA 3.534
degree6 NA 3.449 NA 1.922
degree7 NA 0.797 NA 0.888
Duration Diagnostics
-----------------------
Target Sim Mean Pct Diff Sim SD
edges 25 24.951 -0.196 0.282
Dissolution Diagnostics
-----------------------
Target Sim Mean Pct Diff Sim SD
edges 0.04 0.04 0.113 0plot(dx)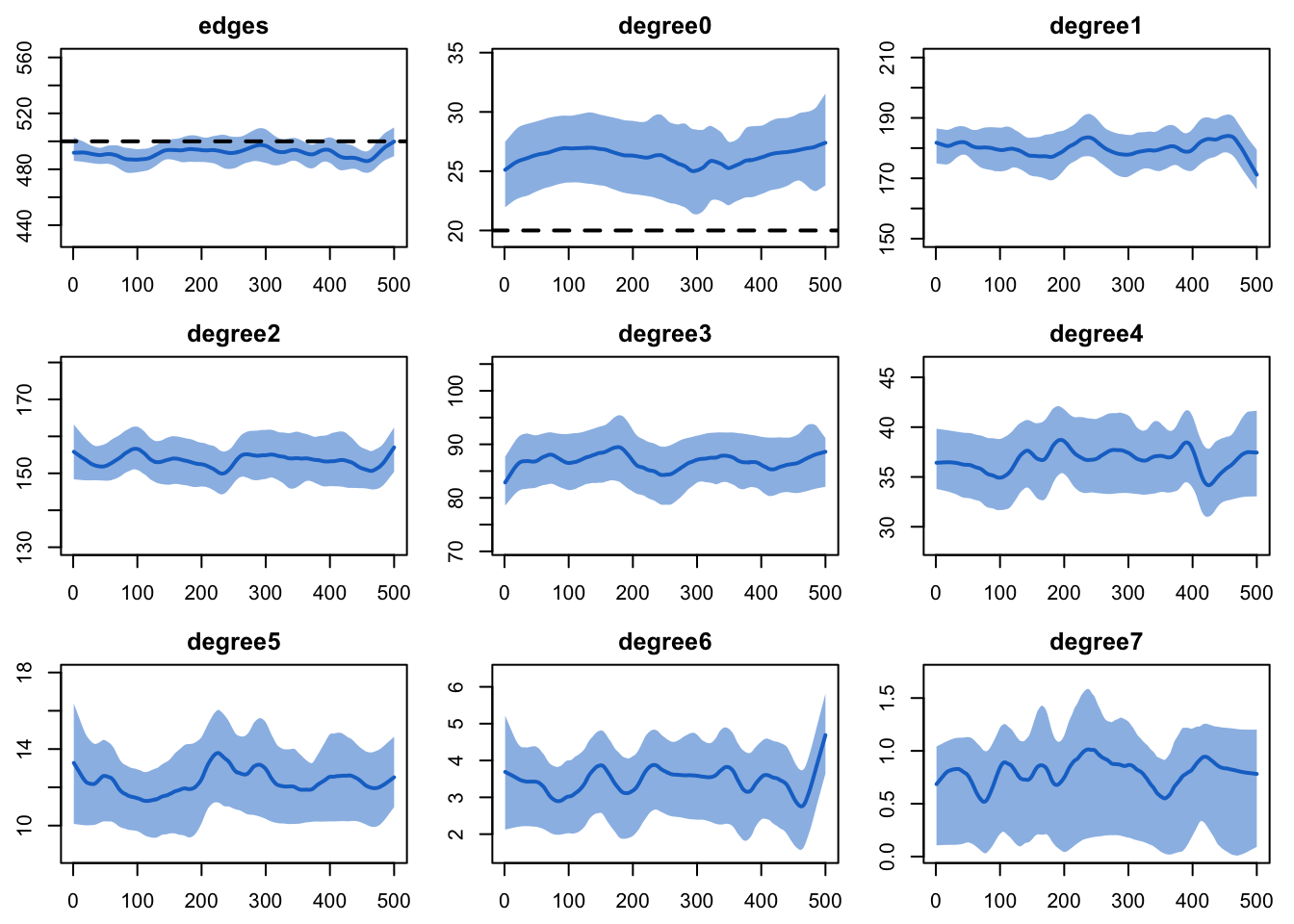
Epidemic Model Parameterization
The epidemic model parameterization for any extension model consists of using the same three input functions as with built-in models.
First we start with the model parameters in param.net.
Note that we have two new rates here: ei.rate and
ir.rate. These control the transitions from E to I, and
then I to R. We have selected these parameter names and input the values
here, but note that the same parameters get pulled into the disease
progression function above. So in general there must be consistency
between the naming of the parameters as inputs and their references in
the model functions.
param <- param.net(inf.prob = 0.5, act.rate = 2,
ei.rate = 0.01, ir.rate = 0.01)Next we specify the initial conditions. Here we are specifying that there are 10 infectious individuals at the epidemic outset. If we wanted to initialize the model with persons only in the latent stage, we would need to set disease status as a nodal attribute on the network instead (following the same approach in the Serosorting Tutorial.
init <- init.net(i.num = 10)Finally, we specify the control settings in control.net.
Extension models like this require some significant updates (compared to
built-in models) here. First, type is set to
NULL in any extension model because we are no longer using
EpiModel to pre-select which modules to run; it is an entirely manual
process. Second, the new input parameter for the
infection.FUN argument is infect; in other
words, our new function for our infection module is the one we built
above. Note that it is called infect. Third, we are
defining a new module, progress.FUN, with an argument that
matches the name of our new progression function. We defined a new
module, rather than replaced the function of the recovery module, just
to show you how to define a new module here. We could have just as
easily done it the latter way with the same results. Finally, we are
explicitly setting resimulate.network to
FALSE; this is the default, so this is not necessary, but
it is just to remind you this is a model without network feedback.
source("d4-s9-SEIR-fx.R")
control <- control.net(type = NULL,
nsteps = 500,
nsims = 1,
ncores = 1,
infection.FUN = infect,
progress.FUN = progress,
resimulate.network = FALSE)In the R scripts that we include with this tutorial, you will see
that we have two separate R script files (for the R Markdown build, they
all go in this single file). One contains the module functions, and the
other contains all the other code to parameterize and run the model. We
do this because it allows for easier interaction with the functions in
browser mode. I will demonstrate this live next. But in general, placing
the functions in a separate file conceptually disentangles the model
functionality from the model parameterization. It is critical, however,
that you source in the file containing the functions before you run
control.net (otherwise, control.net does not
know what infect and progress are).
Epidemic Simulation
Finally we are ready to do the epidemic model simulation and analysis. This is done using the same approach as the built-in models. We will start with running one model in the browser mode interactively.
sim <- netsim(est, param, init, control)And then go back, comment out the browser lines,
re-source the functions, and run a full-scale model with 10
simulations.
source("d4-s9-SEIR-fx.R")
control <- control.net(type = NULL,
nsteps = 500,
nsims = 10,
ncores = 5,
infection.FUN = infect,
progress.FUN = progress,
resimulate.network = FALSE)
sim <- netsim(est, param, init, control)Once the model simulation is complete, we can work with the model object just like a built-in model. Start by printing the output to see what is available.
print(sim)EpiModel Simulation
=======================
Model class: netsim
Simulation Summary
-----------------------
Model type:
No. simulations: 10
No. time steps: 500
No. NW groups: 1
Fixed Parameters
---------------------------
inf.prob = 0.5
act.rate = 2
ei.rate = 0.01
ir.rate = 0.01
groups = 1
Model Functions
-----------------------
initialize.FUN
resim_nets.FUN
infection.FUN
nwupdate.FUN
prevalence.FUN
verbose.FUN
progress.FUN
Model Output
-----------------------
Variables: s.num i.num num ei.flow ir.flow e.num r.num
se.flow
Networks: sim1 ... sim10
Transmissions: sim1 ... sim10
Formation Diagnostics
-----------------------
Target Sim Mean Pct Diff Sim SD
edges 500 492.117 -1.577 17.432
degree0 20 25.781 28.905 5.184
Dissolution Diagnostics
-----------------------
Target Sim Mean Pct Diff Sim SD
edges 0.04 NaN NaN NAHere is the default plot with all the compartment sizes over time. This includes the new summary statistics we tracked in the disease progression function.
par(mar = c(3, 3, 1, 1), mgp = c(2, 1, 0))
plot(sim)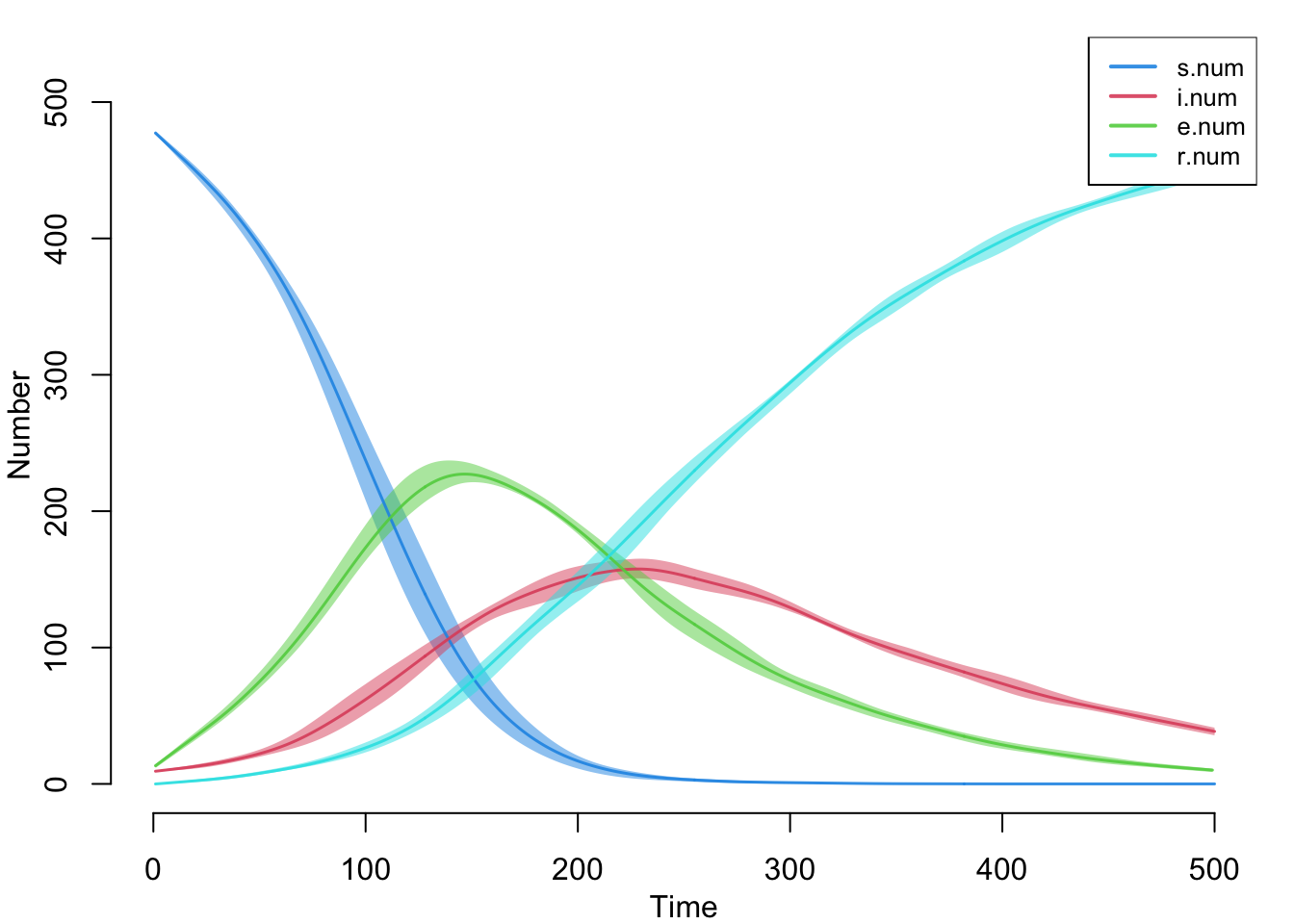
Here are the flow sizes, including the new se.flow
incidence tracker that we established in the new infection function.
plot(sim, y = c("se.flow", "ei.flow", "ir.flow"), legend = TRUE)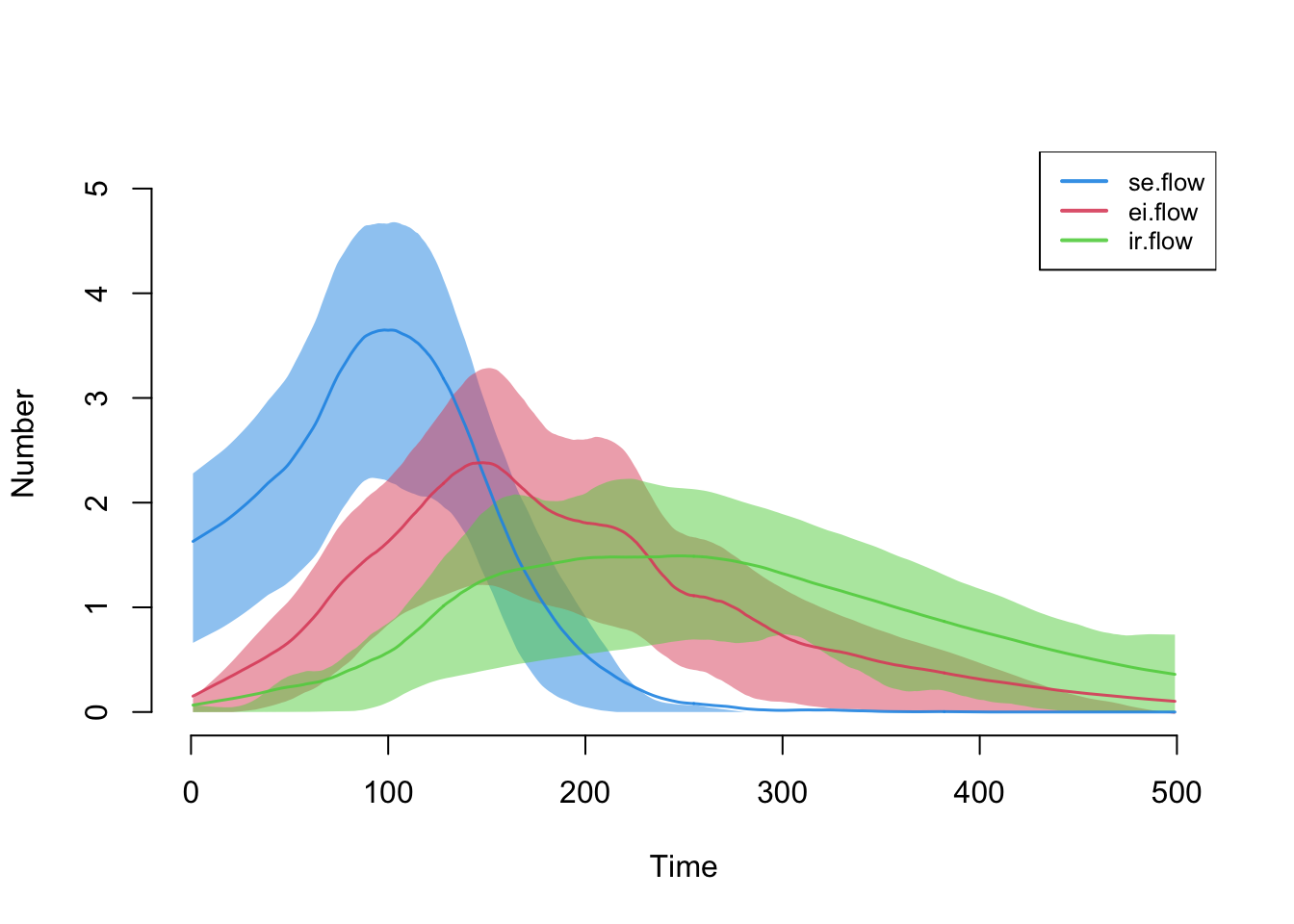
Finally, here is the data frame output from the model, with rows limited to time step 100 across all 10 simulations.
df <- as.data.frame(sim)
df[which(df$time == 100), ] sim time s.num i.num num ei.flow ir.flow e.num r.num se.flow
100 1 100 208 77 500 2 0 187 25 3
600 2 100 220 69 500 1 1 187 22 2
1100 3 100 186 72 500 3 3 206 33 3
1600 4 100 297 40 500 2 1 134 26 3
2100 5 100 210 67 500 0 0 188 31 4
2600 6 100 256 55 500 3 1 162 21 6
3100 7 100 308 38 500 1 1 133 17 4
3600 8 100 246 58 500 0 0 166 26 4
4100 9 100 178 81 500 2 0 205 29 7
4600 10 100 248 52 500 1 3 167 30 3And here are the transmission matrices and their phylogram plots (one per infection seed).
tm1 <- get_transmat(sim)
plot(tm1)found multiple trees, returning a list of 10phylo objects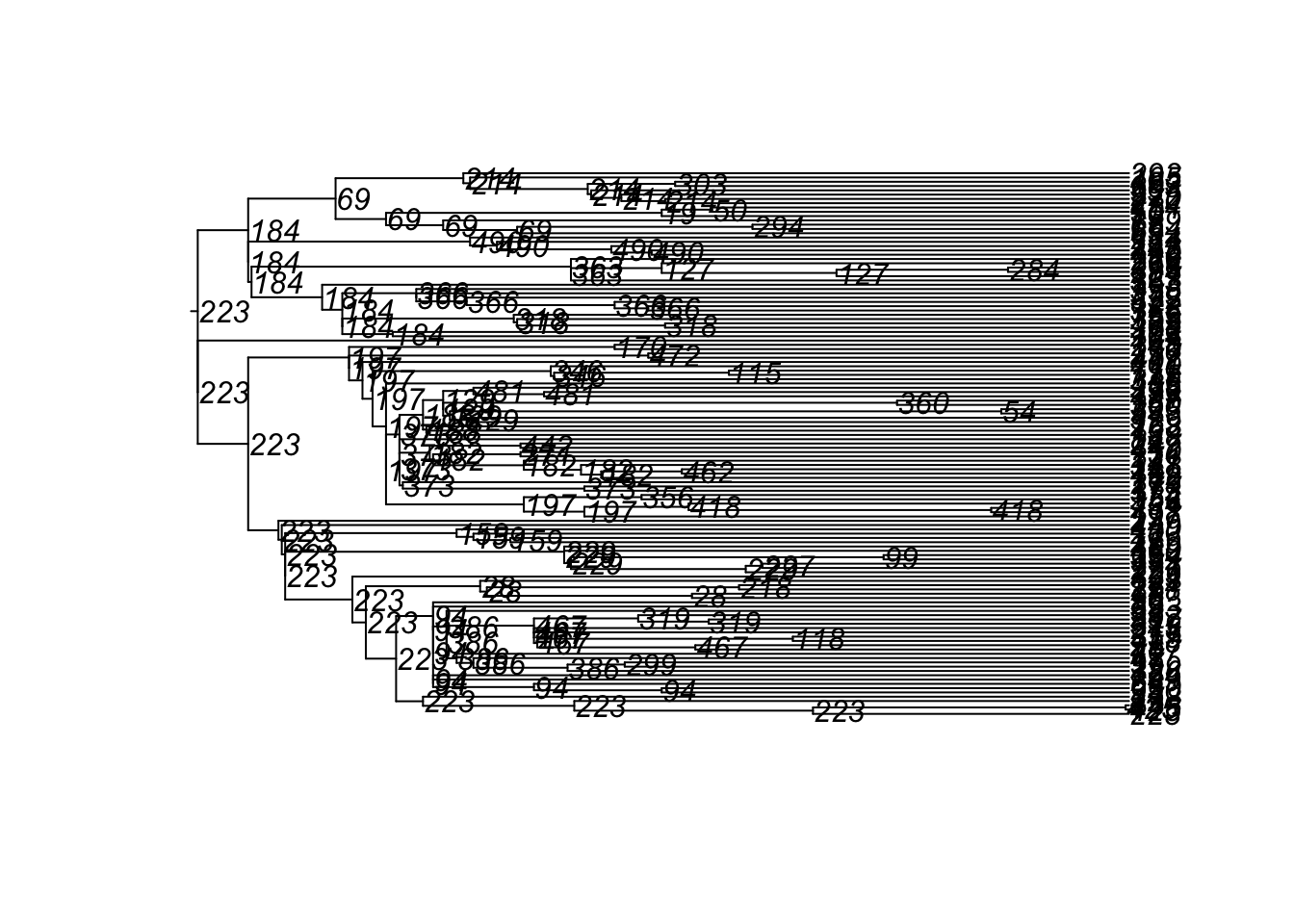
Last updated: 2022-07-07 with EpiModel v2.3.0